 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
New Member
Join Date: Jan 2013
Posts: 6
Rep Power: 13  |
I am trying to simulate the chemically reacting flow> And I found that the formula of reactive NS always gives the negative mass fraction (for product) or over unity mass fraction (for reactant), if the mass diffusion is taken into account. For instance, in 1-d steady case.
Be grateful to any body could give me some hint. |
|
|
|

|
|
|
|
|
#2 |
|
Senior Member
Join Date: Dec 2011
Location: Madrid, Spain
Posts: 134
Rep Power: 15  |
Hi, this is usually due to the numerical dispersion associated to your numerical scheme. This kind of schemes lead to undershoots and overshoots. If you are mixing, say, pure fuel with air then the code will encounter a pretty stiff gradient at the interface, which will result in some wiggles as the fuel diffuses into the oxidiser (Yfuel>1 and Yox<0).
Are you using a centered scheme? Try then using a higher order scheme with lower numerical dispersion. I hope it helps. Cheers, Michujo. |
|
|
|

|
|
|
|
|
#3 | |
|
New Member
Join Date: Jan 2013
Posts: 6
Rep Power: 13  |
Quote:
Thank you for your answer. I believe what you said is quite often based on your experience, and I appreciate that. But what I am facing is the kind of like the cold boundary difficulty (e.g. described in 'Combustion Theory' [F. A. William]). Let me try to clarify this problem. We have mass fraction Y and let's just talk about reactant. Then from species eqn. (1-d, steady state), we have,: dY/dx-(D/V)d_sup{2}Y/dx_sup{2}=w, where D, V and w are diffusion coefficient, velocity and reaction rate respectively. For the reactant, RHS w must be <0 and then based on the eqn above, the calculated Y must be larger than original value. This is my confusion. In MAdding.... |
||
|
|

|
||
|
|
|
#4 |
|
Senior Member
Join Date: Dec 2011
Location: Madrid, Spain
Posts: 134
Rep Power: 15  |
Hi, I misunderstood your question.
As for the cold boundary difficulty I quote C.L. Law's book "Combustion Physics", page 249.: "The cold boundary difficulty can be removed by simply freezing the chemical reaction at a distance sufficienty far upstream of the flame. Such an approach can be interpreted on the basis of an ingnition temperature, 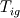 . That is, we simply set . That is, we simply set 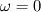 for for 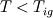 ." ."This way you're supposed to remove the ill-posedness of the species transport equation at the cold boundary (where both dY/dx and d2Y/d2x are zero, so that w should be zero as well, but it is not unless you explicitly freeze it). You might have a go at it and see if it works, although I wonder if you'll just be transporting the ill-posedness somewhat downstream ..., no idea. Cheers, Michujo. |
|
|
|

|
|
|
|
|
#5 | |
|
New Member
Join Date: Jan 2013
Posts: 6
Rep Power: 13  |
Quote:
|
||
|
|

|
||
|
|
|
#6 |
|
New Member
Join Date: Jan 2013
Posts: 6
Rep Power: 13  |
Any big guy could tackle this problem further?
|
|
|
|

|
|
|
|
|
#7 |
|
New Member
Join Date: Jan 2013
Posts: 6
Rep Power: 13  |
up up. hope there are people could help
|
|
|
|

|
|
 |
| Tags |
| negative mass fraction |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| mass flow in is not equal to mass flow out | saii | CFX | 12 | March 19, 2018 05:21 |
| Radiation interface | hinca | CFX | 15 | January 26, 2014 17:11 |
| Water subcooled boiling | Attesz | CFX | 7 | January 5, 2013 03:32 |
| error message | cuteapathy | CFX | 14 | March 20, 2012 06:45 |
| On the damBreak4phaseFine cases | paean | OpenFOAM Running, Solving & CFD | 0 | November 14, 2008 21:14 |