
 |
Multi-step combustion mechanism
Hi Foamers,
I'm working on a simulation of combustion in porous media with reactingFoam solver. in example methane one step mechanism is as follows ____ species ( O2 H2O CH4 CO2 N2 ); reactions { methaneReaction { type irreversibleArrheniusReaction; reaction "CH4 + 2O2 = CO2 + 2H2O"; A 5.2e16; beta 0; Ta 14906; } } ---- in reactins file in constant folder. But I want to use methane from a 4-step mechanism. reactions Ar b Ts CH4 + 1 O2 → CO + 2H2 7.82 . 1013 0 CH4 + H2 O → CO + 3H2 3.00 ∑ 1011 0 H2 + 1 O2 → H2 O 5.0 ∑ 1020 -1 CO + H2 O ---> CO2 + H2 2.75 ∑ 1012 0 Who knows how to put these reactins? comment: i want to use "chemistryReader foamChemistryReader;"in file thermophysicalProperties. and i know to use in chemkin import( "$FOAM_CASE/chemkin/chem.inp") for multi step reaction but i want to use foam chemistry reader. |
it is easy, you need to repeat similar structure for multi-step combustion
means Quote:
|
Quote:
then i should initialize every speice in the reaction ,such as CO,OH....., if so ,how should i initialize them? Thank you!! Best regards, Wen |
GRI: CH4 combustion strange observation
Quote:
Greetings! I wanted to ask if you were successful in implementing the 4-step methane combustion reaction. It does not work for me. I do not understand how to make it work. Can you please help me. Also the tutorial on gri: CH4 combustion, 53 species, 325 reactions. I observe a strange behavior. When I start with an initial temperature of 990K, I obtain the final temperature = 1052.78K after 0.07s However when I start with 991K , I obtain the final temperature =2667.19Kafter 0.07s. I don't understand this sudden change. Do you have any idea for this behavior ? Thanks, Ali |
wenxu, you can copy e.g. 0/O2 file to 0/OH and change initial and boundary values according to your physical model. Or it's possible to just create one file 0/Ydefault for all intermediate and final products of reactions with initialValue uniform 0 and zero values on inlet BCs.
flowAlways, you should have in mind the fact, that Arhenius-type reaction rate has exponent dependence of temperature: if there is enough internal energy in system, reactions will proceed very quickly. Anyway, hitting in that interval is very funny. But it can be checked: try with T=989..992[K] for example. If a result with T=989[K] is similar to T=990[K] and T=992[K] is similar to T=991[K], it's alright. |
Thanks Artem,
I tried with the temp range you said. I obtain 989K(initial) - 1024.88K(at t = 0.07s) 990K(initial) - 1052.78K(at t = 0.07s) 991K(initial) - 2667.19K(at t = 0.07s) 992K(initial) - 2658.16K(at t = 0.07s) 1000K(initial) - 2660.57K(at t=0.07s) What you said makes sense. But I don't understand what is special about 990-991K? Does it have to do with the species reacting or the reaction mechanism ? I am a math student and have no good chemistry knowledge. I will try to understand more about the Arrhenius-type reactions. Thanks! |
Now, i know how to manipulate multi-step reactions. But as far as i know, there is no multi-step combustion models built in OF.
Now i have compiled the EBU model succeed and i design i case to use this model. (i almost substitute the EBU model for the chemistry class. that's means when laminar calss calculate the reaction Rate R(Yi), then it will use the new class EBU to calculate the R(Yi). ) ANY ideas can give me? Thank you!!!! best Regards, wen |
Yeeea, combustion is the very tricky thing, especially turbulent one. I don't know much about OpenFOAM standart turbulent combustion models.
There is flamelet approach for turbulent premixed combustion (in some OF-ext project). Don't know what kind of mathematical model is used in dieselFoam, but physics implies that there is turbulent diffusion combustion with multistage reaction mechanism. In the paper [1] authors use ![W_t = \min[W_{kin}; W_{mix} ] W_t = \min[W_{kin}; W_{mix} ]](/Forums/vbLatex/img/3bb22d9eb9905023845da7388b599487-1.gif) for each reaction of the whole mechanism, but it will be hard to solve ODE system with for each reaction of the whole mechanism, but it will be hard to solve ODE system with 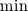 function. function.The problem with EBU is that it can't be used properly in many cases without being limited by kinetic reaction rate, because: 1) in low temperature region there is very-very slow reaction (by physics, not enough energy to break molecules), but EBU will predict combustion there; 2) in regions with fast mixing ( 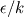 ) EBU can show very fast reaction rate. But! Fast mixing means that in a cell premixed combustion will occur (all components mixed on molecular level before reacting). And premixed combustion rate is limited by kinetic reaction rate. ) EBU can show very fast reaction rate. But! Fast mixing means that in a cell premixed combustion will occur (all components mixed on molecular level before reacting). And premixed combustion rate is limited by kinetic reaction rate.1. Fureby C., Lofstrom C. LES of bluff-body stabilized flames // 25th Symposium on combustion, 1994. |
Dear,Artem Shaklein;
Yeah, i do know EBU does not do well, but that's simple in mathematical form, i want to start from here.(i also want to use the flamelet approach and validate it). Thank you very much, you are so nice and kind. Nice to meet you! PS: i also want to know how to find the chem.inp and thermo.dat files when we implement multi-step reactions. Now i use the thermo.dat from this link. http://combustion.berkeley.edu/gri-m...0/thermo30.dat and chem.inp i use the data from other people's thesis searched from web. Do you know any other resources can i get the two files? PPS: Now i calculate the reactions about CH4 with two steps,the intermediate specie is CO. Thank you again. regards, wen |
hello, wenxu.
look here cfd-online.com/Forums/openfoam/84031-about-chemkintofoam-2.html There is GriMech3 mechanism (for methane combustion) and thermodynamic data. About EBU implementation - I have to think about it. |
Hello wenxu,
If you want to use flamelet model for combustion, just for your information, there are already some implementations available. Check these links 1) http://www.cfd-online.com/Forums/ope...opensmoke.html. 2) http://www.openfoamwiki.net/index.php/Extend-bazaar/solvers/combustion/flameletFoam. I have ported the flameletFoam (the second link) form OpenFoam-2.1 to OpenFoam-2.3, you can find the new version here: http://www.cfd-online.com/Forums/ope...m-2-3-0-a.html Best, Likun |
Thank you,Likun.I will implement theflamelet model next week (then i am thirst for your suggestion probably ), Now the EBU "almost" works for me and there is some problem should be solved. Thank you again,very kind of you!
best, wen |
The combustion models(PaSR,diffusion,FSD) built in the OF all derived from singleStepReaction, these models can be used with muti-step combustion?
regards, wen |
Hello,Likun,
Now i want to implement flamelet model to my case using LES.(mutiphase combustion). Which one of these is better ? And i already install this one and it works for me (Thank you for your work!)http://www.openfoamwiki.net/index.ph.../flameletFoam. But this first one libOpenSMOKE seems more powerful?(sorry, i did not look into it, so this may not be true.) But i can not download it in china, and i do not know why? I really want to validate this one, and have a compare with the flameletFoam? Please give me some suggestion? regards, wen |
Hi Wen,
Nice to hear that the code is working for you. To be honesty, I have also never really looked into the libOpenSMOKE, so I don't really familiar with it, but the post for libOpenSMOKE have lots of very nice information, it will be definitely very helpful to understand the code. But if you are planing to do LES, then maybe the flameletFoam is a better choice, because it is already capable to do LES simulation. If you need, I can send the libOpenSMOKE code to you. Best, Likun |
Dear Likun,
Sorry for the late reply. I really want to have a try of the libOpenSMOKE, it sounds reallly nice. Also the falmeletFOAM is the better choose for me if i do not to add much to the code(I want to use flamelet to simulate coal combusiton). I want to have some opinion of the libOpenSMOKE so if you are free, please send to my Email:981588592@qq.com Thank you very much! The internet speed in china is really slow!!! regards, wenxu |
Already sent.
|
Quote:
|
Hi,Howar,
That's not difficult to simulate gas mixture combustion with flameletFoam. If you want use SLF, then you can keep combustion model not changed. Just generate a new flamelet library with your gas mixtures instead of CH4 with the released version. Flamelet equations can be solved with cantera which in the released version or FlameMaster. regards, wenxu |
Quote:
|
Quote:
Why do you say that Laminar and PaSR combustion models are not for multi-step combustion? Actually I have a problem simulating constant volume combustion using reactingFoam. My geometry is a box with ignition at the center and a 4 step mechanism of CH4 & air is implemented. It's filled with CH4 and air initially. Turbulence model is k-epsilon and I'm testing laminar and PaSR combustion model. It turns out that laminar works pretty good but PaSR doesn't work as what I expect. So could you pls help me figure out my problem? Sihan |
Hi Sehan,
You are correct, infact the Chalmers PaSR model is very similar to the successful EDC approach by Magnussen. It takes into account both the turbulent time scale and the chemical time scale while determining the reaction rate. The chemical timescale is determined by solving the reaction system’s ODEs fully coupled, and finding the characteristic time for that system. So it is meant to take into account a multi-step reaction mechanism. You can find more about PaSR here http://www.tfd.chalmers.se/~valeri/book-chapt7.pdf http://powerlab.fsb.hr/ped/kturbo/op...olmPhD2008.pdf http://web.ist.utl.pt/jose.neves.rod...2011-FINAL.pdf In the PaSR model the reaction rate is determined as reactionRate_PaSR = kappa*reactionRate_laminar kappa ~ 1 when t_chemical >> t_mix so your results with laminar and PaSR will be similar only when the rate is kinetics controlled and not mixing controlled(that is when the flow is laminar and reactions are slow). what is your reaction mechanism? Can you maybe send your case to me ? |
1 Attachment(s)
Quote:
Thanks for your explanation. My problem is I keep adding energy, but the reaction rate is too small when I use PaSR model. Pls find my case in the attachment. --Sihan |
Quote:
|
Quote:
|
ReactingFoam maybe the choice for non-premixed flame. Just add reactions in the reaction dictionary.
Regards, Wen |
Hi Shian,
May you upload your 4-step reaction mechanism of CH4? I try to find it. thank you. Tom |
| All times are GMT -4. The time now is 01:21. |