
 |
interCondensatingEvaporatingFoam
Hello!
I'm new in OpenFoam and use the interCondensatingEvaporatingFoam solver. The tutorial condensatingVessel slightly confuses me. Of course the material values depend on the temperature. The vapour values are given for T=386, its the same like the initial value of the temperature, so that sounds logical. But I don't understand which temperature is assigned to the liquid phase (I think ca. 330K, because of the values from FC-72) and why? An other question is which Prandtl Number I should use? And the last question: The saturation temperature in the thermophysicalProperties is given to T=366 K. I checked this and found for FC-72 a T_Sat=330K. It is a mistake or is there a deeper meaning behind it? I hope anybody can help me to understand this solver. Regards Robin |
Quote:
The "setFields" utility is probably called by that tutorial in "Allrun". There is an associated "./system/setFieldsDict" in which you can overwrite the initial condition for all fields with a different value in a specific region. That's also how they add a water column to the "damBreak" tutorial. Quote:
Quote:
|
Thank you for your response, but I think I must specify my concern.
interCondensatingEvaporatingFoam is a relativ new solver from OpenFoam v1606+. The tutorial is about a vessel which is filled with gas and then you have a negativ heat flow so that the temperature drops and the gas begins to condense. The material values are for the gas phase as well as for the liquig phase from the transportPorperties. But I don't understand why exactly these material values are used for the liquid phase. The inital pressure is given to 1bar. Out of this value I have checked the saturation temperature and find the value T=330K... Maybe I make a big mistake. |
The bottle is open at the top.
Of course you're right, I can change the saturation temperature. I found it just a little strange. The issue that confuses me more is my first question: the liquid phase material values in the transportProperties. Do you have any ideas about this? |
Quote:
Since you have a problem with a cooling gas, the gas is at the varying temperature, and therefore the liquid may be assumed to be at the saturation temperature. Therefore, I reckon the liquid properties are taken at the saturation temperature. In your first post you estimated the temperature of the properties to be 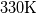 , and you found the saturation temperature to be equal to , and you found the saturation temperature to be equal to 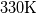 , hence this seems correct. , hence this seems correct.The saturation temperature was, however, set to a higher value, but as we had already concluded: that seems to be inconsistent/incorrect. |
Ok, that sounds logical.
Thanks a lot for your help, that makes me a big step forward! |
| All times are GMT -4. The time now is 16:50. |