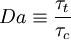Damkohler number
From CFD-Wiki
| (3 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
This regime is the "well-stirred reactor", where products and reactants are rapidly mixed. | This regime is the "well-stirred reactor", where products and reactants are rapidly mixed. | ||
The [[Karlovitz number]] is linked to the Damkholer number. | The [[Karlovitz number]] is linked to the Damkholer number. | ||
| + | |||
| + | |||
| + | The Damkohler number is also defined in a chemical non-equilibrium process at very high velocities of the fluid or vehicles (with reference to fluid). There are two ratios of Damkohler number that can be defined and are as follows: | ||
| + | |||
| + | 1. First Damkohler Number or DAM 1 | ||
| + | |||
| + | <math> | ||
| + | DAM 1 = \frac{t_{res}}{\tau} | ||
| + | </math> | ||
| + | |||
| + | Where <math> t_{res} </math> is the residence time of the flow, where <math> \frac{L_{ref}}{U_{ref}} </math> where <math> L_{ref} </math> is the reference length of the vehicle and <math> U_{ref} </math> is the reference velocity of fluid or vehicle with respect to fluid | ||
| + | |||
| + | |||
| + | 2. Second Damkohler Number or DAM 2 | ||
| + | |||
| + | <math> | ||
| + | DAM 2 = \frac{q_{ne}}{h_o} | ||
| + | </math> | ||
| + | |||
| + | Where <math> q_{ne} </math> is the energy involved in a non-equilibrium process and <math> h_o </math> is the total enthalpy of the flow | ||
[[Category:Dimensionless parameters]] | [[Category:Dimensionless parameters]] | ||
Latest revision as of 13:50, 6 February 2012
The Damkohler number is used in turbulent combustion
and corresponds to the ratio of chemical time scale 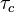 and turbulent time scale
and turbulent time scale 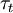 .
This turbulent scale is usually taken as the integral scale.
.
This turbulent scale is usually taken as the integral scale.
Damkohler number measures how important is the interaction between chemistry and turbulence. Most combustion models are placed in the extremes of Damkohler.
If 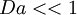 the turbulence is much faster than the chemistry.
This regime is the "well-stirred reactor", where products and reactants are rapidly mixed.
The Karlovitz number is linked to the Damkholer number.
the turbulence is much faster than the chemistry.
This regime is the "well-stirred reactor", where products and reactants are rapidly mixed.
The Karlovitz number is linked to the Damkholer number.
The Damkohler number is also defined in a chemical non-equilibrium process at very high velocities of the fluid or vehicles (with reference to fluid). There are two ratios of Damkohler number that can be defined and are as follows:
1. First Damkohler Number or DAM 1
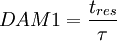
Where 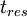 is the residence time of the flow, where
is the residence time of the flow, where 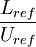 where
where 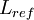 is the reference length of the vehicle and
is the reference length of the vehicle and 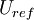 is the reference velocity of fluid or vehicle with respect to fluid
is the reference velocity of fluid or vehicle with respect to fluid
2. Second Damkohler Number or DAM 2
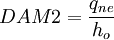
Where 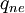 is the energy involved in a non-equilibrium process and
is the energy involved in a non-equilibrium process and 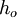 is the total enthalpy of the flow
is the total enthalpy of the flow