Combustion
From CFD-Wiki
m (→LES equations) |
(→Governing Equations for Reacting Flows) |
||
| Line 172: | Line 172: | ||
\right) | \right) | ||
</math> | </math> | ||
| + | |||
| + | [[Category:Equations]] | ||
== Infinitely fast chemistry == | == Infinitely fast chemistry == | ||
Revision as of 15:42, 14 November 2005
What is combustion -- Physics versus modelling
Combustion phenomena consists of many physical and chemical processes with broad range of time scales. Mathematical description of combustion is not always trivial. Analytical solutions exists only for basic situations of laminar flame and because of its assumptions it is often restricted to few problems solved usually in zero or one-dimensional space.
Problems solved today concern mainly turbulent flows, gas as well as liquid fuels, pollution issues (products of combustion as well as for example noise pollution). These problems require not only extensive experimental work, but also numerical modelling. All combustion models must be validated against the experiments as each one has its own drawbacks and limits. However here the modelling part will be mainly addressed.
Reaction mechanisms
The combustion is mainly chemical process and although we can, to some extend, describe flame without any chemistry informations, for modelling of flame propagation we need to know the speed of reactions, product concentrations, temperature and other parameters. Therefore more or less detailed information about reaction kinetics is essential for any combustion model. Mixture will generally combust, if the reaction of fuel and oxidiser is fast enough to maintain until all of the mixture is burned into products. If the reaction is too slow, the flame will extinguish, if too fast, explosion or even detonation will occur. The reaction rate of typical combustion reaction is influenced mainly by concentration of reactants, temperature and pressure.
A stoichiometric equation of an arbitrary equation can be written as:
|
|
where $\nu$ is the stoichiometric coefficient, 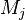 is arbitrary species. One
prime specifies the reactants and double prime products of the reaction.
is arbitrary species. One
prime specifies the reactants and double prime products of the reaction.
Reaction rate, expressing the rate of disappearance of reactant i of such a reaction, is defined as:
|
|
in which k is the specific reaction rate constant. Arrhenius found that this constant is a function only of temperature and this function is defined as:
|
|
where A is pre--exponential factor, E is activation energy and 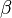 is
temperature exponent.
These constants for given reactions can be found in literature.
The reaction mechanism can be given from experiments for every reaction
resolved, it could be also constructed numerically by automatic generation
method (see [Griffiths (1994)] for review on reaction mechanisms).
For simple hydrocarbon tens to hundreds of reactions are involved.
By analysis and systematic reduction of reaction mechanisms global reaction
(from one to five step reactions) can be found (see [Westbrook (1984)]).
is
temperature exponent.
These constants for given reactions can be found in literature.
The reaction mechanism can be given from experiments for every reaction
resolved, it could be also constructed numerically by automatic generation
method (see [Griffiths (1994)] for review on reaction mechanisms).
For simple hydrocarbon tens to hundreds of reactions are involved.
By analysis and systematic reduction of reaction mechanisms global reaction
(from one to five step reactions) can be found (see [Westbrook (1984)]).
Governing Equations for Reacting Flows
Together with the usual Navier-Stokes for compresible flows (See Governing Equations), additional equations are
needed in reacting flows.
The mass fraction transport equation for k-th species 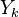 is:
is:
where Ficks law is assumed for scalar diffusion with 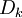 , the species difussion coefficient and
, the species difussion coefficient and 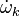 is the species reaction rate.
A non-reactive scalar (like the mixture fraction
is the species reaction rate.
A non-reactive scalar (like the mixture fraction 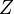 ) had the following transport equation:
) had the following transport equation:
where 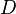 is the diffusion coefficient of the passive scalar.
is the diffusion coefficient of the passive scalar.
RANS equations
In turbulent flows, Favre averaging is often used and the mass fraction transport equation is transformed to
where the turbulent fluxes 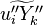 and reaction terms
and reaction terms
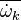 needs to be closed.
needs to be closed.
The passive scalar turbulent transport equation is
where similar to the mass fraction equation, 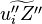 needs modelling.
needs modelling.
In addition to the mean passive scalar equation,
an equation for the Favre variance 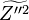 is often employed
is often employed
where 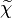 is the mean scalar dissipation rate
defined as
is the mean scalar dissipation rate
defined as 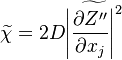 This term and the variance diffusion fluxes needs to be modelled.
This term and the variance diffusion fluxes needs to be modelled.
LES equations
The Large eddy simulation (LES) equation for reactive flows introduces equations for the filtered species mass fractions to the compressible flow field. Similar to #RANS equations, but using Favre filtering instead of Favre averaging. The filtered mass fraction transport equation is
where 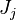 is the transport of subgrid fluctuations of mass fraction
is the transport of subgrid fluctuations of mass fraction
and has to be modelled. Fluctuations of diffusion coefficients are often ignored and their contributions much smaller than apparent turbulent diffusion due to transport of subgrid fluctuations. The first term on the right hand side is then
Infinitely fast chemistry
All combustion models can be divided into two main groups according to the assumptions on the reaction kinetics. We can either assume the reactions to be infinitely fast - compared to e.g. mixing of the species, or of the comparable time scale of the mixing process. The simpler approach assuming chemistry fast enough, that the limiting process is mixing of the species is historically older approach and even today can be appropriate approach. It is simpler to solve then #Finite rate chemistry models, but introduces errors to the solution which may or may not be important.
Premixed Combustion
Premixed flame occurs in mixtures of fuel and oxidiser, homogeneously premixed prior to the flame. These flames are not limited only to gas fuels, but also to the pre-vaporised fuels. Typical example of premixed laminar flame is bunsen burner, where the air enters the fuel stream. The mixture burns in the wake of the riser tube walls forming nice stable flame. The premixed flames has many advantages in terms of control of temperature and products and pollution concentration, but introduce also some dangers like the autoignition (in the supply system).
Turbulent flame speed model
Eddy Break-Up model
The Eddy Break-Up model is the typical example of mixed-is-burnt combustion model. It is based on the work of Magnussen and Hjertager, and Spalding and can be found in all CFD packages. The model assumes the reactions to be completed in the moment of mixing, so that the reaction rate is completely controlled by turbulent mixing. The combustion is described by a single step global chemical reaction:
|
|
in which F stands for fuel, O for oxidiser and P for products of the reaction. Alternativelly we can have multistep scheme, where each reaction has its own mean reaction rate. The mean reaction rate is given by:
|
|
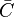 denotes mean concentrations for fuel, oxidiser and products
respectively, A and B are model constants with typical values of 0.5
and 4.0 respectively. The values of these constants are fitted according
to the experimental results and they are suitable for most of the general cases.
Still they are just constants based on experimental fitting and they need not
be suitable for all the situations.
Care must be taken especially in highly strained regions, where the ratio of
denotes mean concentrations for fuel, oxidiser and products
respectively, A and B are model constants with typical values of 0.5
and 4.0 respectively. The values of these constants are fitted according
to the experimental results and they are suitable for most of the general cases.
Still they are just constants based on experimental fitting and they need not
be suitable for all the situations.
Care must be taken especially in highly strained regions, where the ratio of 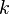 to
to  is large (flame-holder wakes, walls ...). In those regions a positive reaction rate occurs and an artificial flame can be observed.
CFD codes usually has some remedies to overcome this problem.
is large (flame-holder wakes, walls ...). In those regions a positive reaction rate occurs and an artificial flame can be observed.
CFD codes usually has some remedies to overcome this problem.
This model largely over-predicts temperatures and concentrations of species like CO and other species. Still this model is quite popular for its simplicity and relatively easy convergence and implementation.
Bray-Moss-Libby Model
Non premixed combustion
Conserved scalar equilibrium models
Finite rate chemistry
Premixed Combustion
Coherent Flame Model
Flamelets based on G equation
Non-premixed Combustion
Flamelets based on conserved scalar
Peters (2000) define Flamelets as "thin diffusion layers embedded in a turbulent non-reactive flow field".
If the chemistry is fast enough, the chemistry is active within a thin region
where the chemistry conditions are in (or close to) stoichiometric conditions, the "flame" surface.
This thin region is assumed to be smaller than Kolmogorov length scale and therefore the
region is locally laminar. The flame surface is defined as an iso-surface of a certain scalar 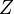 ,
mixture fraction in non-premixed combustion.
,
mixture fraction in non-premixed combustion.
The reactive problems is therefore split into two parts: First, the mixing , which consists of the location of the flame surface which is a non-reactive problem concerning the propagation of a passive scalar. And second, the flame structure , which deals with the distribution of the reactive species inside the flamelet.
To obtain the distribution inside the flame front we assume it is locally one-dimensional and depends only on time and the scalar coodinate.
Using the following chain rules for the time
and spatial coordinate
to the species transport equation (see #Governing Equations for Reacting Flows) and re-arranging, we obtain
The second and third term in the LHS cancel due to continuity and mixture fraction transport, the equation therefore boils down to
where 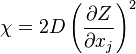 is called the scalar dissipation
and controls the mixing, providing the interaction between the flow and the chemistry.
is called the scalar dissipation
and controls the mixing, providing the interaction between the flow and the chemistry.
If the flame dependence on time is dropped, even though he field 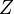 still depends on it.
still depends on it.
This approach is called the Stationary Laminar Flamelet Model (SLFM) and has the advantage that libraries of
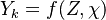 can be pre-computed and stored in look-up tables with all the required complex chemistry.
can be pre-computed and stored in look-up tables with all the required complex chemistry.
Flamelet Computation and Flamelet Libraries
The computation of non-premixed turbulent flames based on laminar-flamelet models is generally based on two-dimensional or three-dimensional CFD codes that employ standard models for fluid-mechanical closure of the govening equations. In many cases, for that purpose standard models such as the k-epsilon model are used, but occasionally more sophisticated models such as Reynolds-Stress models are also employed.
Chemical-source-term closure is a different matter. To this end, the CFD codes carry out suitable averaging procedures, such as pdf-avaraging on the basis of a beta function or a clipped Gaussian distribution. The quantities to be averaged are laminar-flamelet profiles, i.e., results from laminar-flamelet computations. Generally, these flamelet computations are carried oout a-priori, i.e, they are performed separately and prior to the turbulent-combustion simulation with the CFD code. Depending on the specific laminar-flamelet model used for the turbulent-combustion simulation, one or several parameters are varied in the laminat computatations. For instance, if the computations are based on
then the variable parameter is the scalar dissipation rate 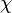 . The flamelet profiles for the various parameter values are stored in a dataset or file which is called
a "flamelet library". For the generation of such libraries ready to use software is avalable such as Softpredict's Combustion Simulation Laboratory COSILAB [1] with its relevant solver RUN1DL, which can be used for a variety of relevant geometries; see various
publications that are available for download.
. The flamelet profiles for the various parameter values are stored in a dataset or file which is called
a "flamelet library". For the generation of such libraries ready to use software is avalable such as Softpredict's Combustion Simulation Laboratory COSILAB [1] with its relevant solver RUN1DL, which can be used for a variety of relevant geometries; see various
publications that are available for download.
Flamelets in turbulent combustion
In turbulent flames the interest is 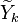 .
In flamelets, the flame thickness is assumed to be much smaller than Kolmogorov scale
and obviously is much smaller than the grid size.
It is therefore needed a distribution of the passive scalar within the cell.
.
In flamelets, the flame thickness is assumed to be much smaller than Kolmogorov scale
and obviously is much smaller than the grid size.
It is therefore needed a distribution of the passive scalar within the cell.
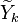 cannot be obtained directly from the flamelets library
cannot be obtained directly from the flamelets library
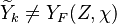 , where
, where 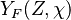 corresponds
to the value obtained from the flamelets libraries.
A generic solution can be expressed as
corresponds
to the value obtained from the flamelets libraries.
A generic solution can be expressed as
where 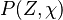 is the joint Probability Density Function (PDF) of the mixture fraction
and scalar dissipation which account for the scalar distribution inside the cell and "a priori"
depends on time and space.
is the joint Probability Density Function (PDF) of the mixture fraction
and scalar dissipation which account for the scalar distribution inside the cell and "a priori"
depends on time and space.
The most simple assumption is to use a constant distribution of the scalar dissipation within the cell and the above equation reduces to
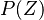 is the PDF of the mixture fraction scalar and simple models (such as Gaussian or a beta PDF)
can be build depending only on two moments of the scalar
mean and variance,
is the PDF of the mixture fraction scalar and simple models (such as Gaussian or a beta PDF)
can be build depending only on two moments of the scalar
mean and variance,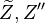 .
.
If the mixture fraction and scalar dissipation are consider independent variables,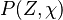 can be written as
can be written as 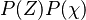 . The PDF of the scalar dissipation is assumed to be log-normal with
variance unity.
. The PDF of the scalar dissipation is assumed to be log-normal with
variance unity.
Conditional Moment Closure (CMC)
In Conditional Moment Closure (CMC) methods we assume that the species mass fractions are all correlated with the mixture fraction (in non premixed combustion).
From Probability density function we have
where 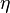 is the sample space for
is the sample space for 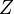 .
.
CMC consists of providing a set of transport equations for the conditional moments which define the flame structure.
Experimentally, it has been observed that temperature and chemical radicals are strong non-linear functions of mixture fraction. For a given species mass fraction we can decomposed it into a mean and a fluctuation:
The fluctuations 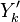 are usually very strong in time and space which makes the closure
of
are usually very strong in time and space which makes the closure
of 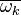 very difficult.
However, the alternative decomposition
very difficult.
However, the alternative decomposition
where 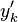 is the fluctuation around the conditional mean or the "conditional fluctuation".
Experimentally, it is observed that
is the fluctuation around the conditional mean or the "conditional fluctuation".
Experimentally, it is observed that 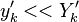 , which forms the basic assumption of the CMC method.
Closures. Due to this property better closure methods can be used reducing the non-linearity
of the mass fraction equations.
, which forms the basic assumption of the CMC method.
Closures. Due to this property better closure methods can be used reducing the non-linearity
of the mass fraction equations.
The Derivation of the CMC equations produces the following CMC transport equation
where 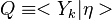 for simplicity.
for simplicity.
In this equation, high order terms in Reynolds number have been neglected. (See Derivation of the CMC equations for the complete series of terms).
It is well known that closure of the unconditional source term
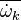 as a function of the
mean temperature and species (
as a function of the
mean temperature and species (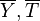 ) will give rise to large errors.
However, in CMC the conditional averaged mass fractions contain more information and fluctuations around the mean are much smaller.
The first order closure
) will give rise to large errors.
However, in CMC the conditional averaged mass fractions contain more information and fluctuations around the mean are much smaller.
The first order closure
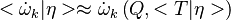 is a good approximation in zones which are not close to extinction.
is a good approximation in zones which are not close to extinction.
Second order closure
A second order closure can be obtained if conditional fluctuations are taken into account.
For a chemical source term in the form 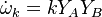 with the rate constant in Arrhenius form
with the rate constant in Arrhenius form
![k=A_0 T^\beta exp [-Ta/T]](/W/images/math/2/e/0/2e0c186caff5787cd5f2f76baac86531.png) the second order closure is (Klimenko and Bilger 1999)
the second order closure is (Klimenko and Bilger 1999)
where 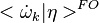 is the first order CMC closure and
is the first order CMC closure and
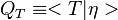 .
When the temperature exponent
.
When the temperature exponent 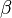 or
or 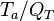 are large the error of taking the first order approximation increases.
Improvement of small pollutant predictions can be obtained using the above reaction
rate for selected species like CO and NO.
are large the error of taking the first order approximation increases.
Improvement of small pollutant predictions can be obtained using the above reaction
rate for selected species like CO and NO.
Double conditioning
Close to extinction and reignition. The conditional fluctuations can be very large
and the primary closure of CMC of "small" fluctuations is not longer valid.
A second variable 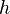 can be chosen to define a double conditioned mass fraction
can be chosen to define a double conditioned mass fraction
Due to the strong dependence on chemical reactions to temperature, 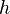 is advised to be a temperature related variable (Kronenburg 2004).
Scalar dissipation is not a good choice, due to its log-normal behaviour
(smaller scales give highest dissipation). A must better choice is the sensible enthalpy
or a progress variable.
Double conditional variables have much smaller conditional fluctuations and allow
the existence of points with the same chemical composition which can be fully burning
(high temperature) or just mixing (low temperature).
The range of applicability is greatly increased and allows non-premixed and premixed problems
to be treated without ad-hoc distinctions.
The main problem is the closure of the new terms involving cross scalar transport.
is advised to be a temperature related variable (Kronenburg 2004).
Scalar dissipation is not a good choice, due to its log-normal behaviour
(smaller scales give highest dissipation). A must better choice is the sensible enthalpy
or a progress variable.
Double conditional variables have much smaller conditional fluctuations and allow
the existence of points with the same chemical composition which can be fully burning
(high temperature) or just mixing (low temperature).
The range of applicability is greatly increased and allows non-premixed and premixed problems
to be treated without ad-hoc distinctions.
The main problem is the closure of the new terms involving cross scalar transport.
The double conditional CMC equation is obtained in a similar manner than the conventional CMC equations
LES modelling
In a LES context a conditional filtering operator can be defined
and 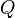 therefore represents a conditionally filtered reactive scalar.
therefore represents a conditionally filtered reactive scalar.
Multiple Mapping Closure (MMC)
Linear Eddy Model
The Linear Eddy Model (LEM) was first developed by Kerstein(1988). It is an one-dimensional model for representing the flame structure in turbulent flows.
In every computational cell a molecular, diffusion and chemical model is defined as
where 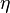 is a spatial coordinate. The scalar distribution obtained can be seen as a
one-dimensional reference field between Kolmogorov scale and grid scales.
is a spatial coordinate. The scalar distribution obtained can be seen as a
one-dimensional reference field between Kolmogorov scale and grid scales.
In a second stage a series of re-arranging stochastic event take place.
These events represent the effects
of a certain turbulent structure of size 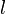 , smaller than the grid size at a location
, smaller than the grid size at a location 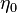 within the one-dimensional domain. This vortex distort the
within the one-dimensional domain. This vortex distort the 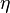 field obtain by the one-dimensional equation,
creating new maxima and minima in the interval
field obtain by the one-dimensional equation,
creating new maxima and minima in the interval 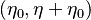 .
The vortex size
.
The vortex size 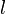 is chosen randomly based on the inertial scale range while
is chosen randomly based on the inertial scale range while
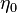 is obtained from a uniform distribution in
is obtained from a uniform distribution in 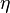 .
The number of events is chosen to match the turbulent diffusivity of the flow.
.
The number of events is chosen to match the turbulent diffusivity of the flow.
PDF transport models
Probability Density Function (PDF) methods are not exclusive to combustion, although they are particularly attractive to them. They provided more information than moment closures and they are used to compute inhomegenous turbulent flows.
PDF methods are based on the transport equation of the joint-PDF of the scalars.
Denoting 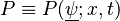 where
where
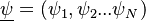 is the phase space for the reactive scalars
is the phase space for the reactive scalars
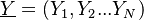 .
The transport equation of the joint PDF is:
.
The transport equation of the joint PDF is:
where the chemical source term is closed. Another term appeared on the right hand side which accounts for the effects of
the molecular mixing on the PDF, is the so called "micro-mixing " term.
Equal diffusivities are used for simplicity 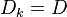
A more general approach is the velocity-composition joint-PDF
with 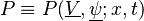 , where
, where
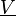 is the sample space of the velocity field
is the sample space of the velocity field
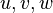 . This approach has the advantage of avoiding gradient-diffusion
modelling. A similar equation to the above is obtained combining the momentum
and scalar transport equation.
. This approach has the advantage of avoiding gradient-diffusion
modelling. A similar equation to the above is obtained combining the momentum
and scalar transport equation.
The PDF transport equation can be solved in two ways: through a Lagrangian approach
using stochastic methods or in a Eulerian ways using stochastic fields.
Lagrangian
The main idea of Lagrangian methods is that the flow can be represented by an ensemble of fluid particles. Central to this approach is the stochastic differential equations and in particular the Langevin equation.
Eulerian
Instead of stochastic particles, smooth stochastic fields can be used to represent the probability density function (PDF) of a scalar (or joint PDF) involved in transport (convection), diffusion and chemical reaction (Valino 1998). This method is purely Eulerian and offers implementations advantages compared to Lagrangian or semi-Eulerian methods. Transport equations for scalars are often easy to programme and normal CFD algorithms can be used (see Discretisation of convective term)
A new set of 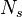 scalar variables
(the stochastic field
scalar variables
(the stochastic field 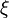 ) is used to represent the
PDF
) is used to represent the
PDF
References
- Griffiths, J. F. (1994), "Reduced Kinetic Models and Their Application to Practical Combustion Systems", Prog. in Energy and Combustion Science,Vol. 21, pp. 25-107.
- Peters, N. (2000), Turbulent Combustion, Cambridge University Press.
- Poinsot, T.,Veynante, D. (2001), Theoretical and Numerical Combustion, ISBN 1-930217-05-6, R. T Edwards.
- Westbrook, Ch. K., Dryer,F. L., (1984), "Chemical Kinetic Modeling of Hydrocarbon Combustion", Prog. in Energy and Combustion Science,Vol. 10, pp. 1-57.

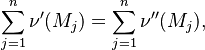
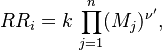
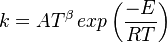
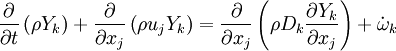
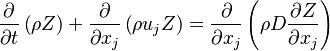
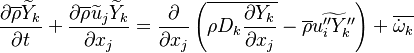
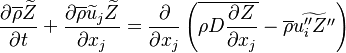
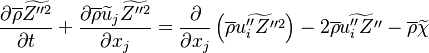
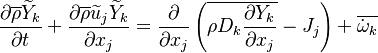
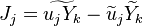
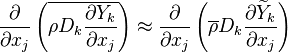
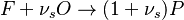
![\bar{\dot\omega}_F=A_{EB} \frac{\epsilon}{k}
min\left[\bar{C}_F,\frac{\bar{C}_O}{\nu},
B_{EB}\frac{\bar{C}_P}{(1+\nu)}\right]](/W/images/math/9/d/6/9d67c48a8dd1f2decb0d5bf3336d215b.png)
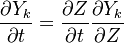
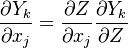
![\rho \frac{\partial Y_k}{\partial t} + Y_k \left[
\frac{\partial \rho}{\partial t} + \frac{\partial \rho u_j}{\partial x_j}
\right]
+ \frac{\partial Y_k}{\partial Z} \left[
\rho \frac{\partial Z}{\partial t} + \rho u_j \frac{\partial Z}{\partial x_j} -
\frac{\partial}{\partial x_j}\left( \rho D \frac{\partial Z}{\partial x_j} \right)
\right]
=
\rho D \left( \frac{\partial Z}{\partial x_j} \frac{\partial Z}{\partial x_j} \right) + \dot \omega_k](/W/images/math/a/c/0/ac05bf8a3070c4ead5af3254ef9c20f9.png)
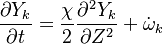
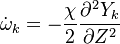
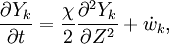
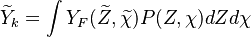
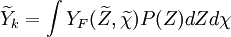
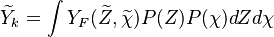
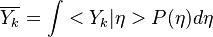
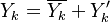
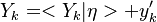
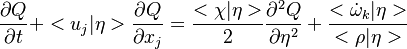
![< \dot \omega_k|\eta> \approx < \dot \omega_k|\eta >^{FO}
\left[1+ \frac{< Y''_A Y''_B |\eta>}{Q_A Q_B}+ \left( \beta + T_a/Q_T \right)
\left(
\frac{< Y''_A T'' |\eta>}{Q_AQ_T} + \frac{< Y''_B T'' |\eta>}{Q_BQ_T}
\right) + ...
\right]](/W/images/math/f/9/c/f9c779360789a63c6bb68e8c13d0489a.png)
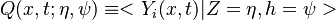
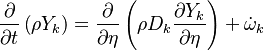
![\frac{\partial <\rho | \underline{Y}=\underline{\psi}> P }{\partial t} + \frac{
\partial <\rho u_j | \underline{Y}=\underline{\psi}> P }{\partial x_j} =
\sum^N_\alpha \frac{\partial}{\partial \psi_\alpha}\left[ \rho \dot{\omega}_\alpha P \right]
- \sum^N_\alpha \sum^N_\beta \frac{\partial^2}{\partial \psi_\alpha \psi_\beta}
\left[ <D \frac{\partial Y_\alpha}{\partial x_i} \frac{\partial Y_\beta}{\partial x_i} | \underline{Y}=\underline{\psi}> \right] P](/W/images/math/1/3/7/13767f4c5947b8df82fdd84c944ea62b.png)
![P (\underline{\psi}; x,t) = \frac{1}{N} \sum^{N_s}_{j=1} \prod^{N}_{k=1} \delta \left[\psi_k -\xi_k^j(x,t) \right]](/W/images/math/f/2/3/f23f3d712f59f83ab17babbfbe9834ab.png)