 |
|
|
[Sponsors] | |||||
Eddy dissipation model temperature profile problem |
 |
|
|
LinkBack | Thread Tools | Search this Thread | Display Modes |
|
|
|
#1 |
|
New Member
Ozan KEKÜL
Join Date: Nov 2020
Posts: 8
Rep Power: 5  |
Hello ,
I'm trying to model NH3-oxy combustion through eddy dissipation model but i m getting too high temperature values. I use one step reaction as NH3+0,75O2 = 0,5N2 + 1,5H2O. I shared the details of my model as follows; 1. Equivalence ratio : 0,83 2. Thermal power: 10 kw 3. Viscous model: k-epsilon 4. Eddy dissipation model 5. mixture materials has been taken from fluent database for nh3 and other species. (i dont know exactly which properties can i change on mixture material to decrease temperature values) 6. Reactions dialog box: rate exponents both for nh3 and o2 are 1 , for products are zero. Mixing rate values A : 4, B: 0.5 ( i have changed the A value from 4 to 1 but no avail) 7. radiation model is P1. Consequently, what should i do to get plausible temperature values for nh3-oxy combustion. your suggestion will be very helpful . Thanks in advance. |
|
|
|

|
|
|
|
|
#2 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
What do you mean by too high? Give a number
|
|
|
|

|
|
|
|
|
#3 |
|
New Member
Ozan KEKÜL
Join Date: Nov 2020
Posts: 8
Rep Power: 5  |
||
|
|

|
|
|
|
|
#4 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
You know, you are using pure oxygen as oxidizer if I understand well, right? That is, there’s no nitrogen in the combustion chamber except as combustion product? If yes, I am not sure that it does not make sense. For air/nh3 i would expect something on the order of 2000K, especially if you are not too lean, but for pure oxygen, this might be way higher. On top of that, eddy dissipation overpredicts (~200K) the flame temperature
The temperature contour you posted also seems very reasonable in terms of shape, so I expect you to have done nothing too wrong in the setup. Could not find adiabatic flame temperatures data for pure oxygen combustion but it would not surprise me if it is around 3k |
|
|
|

|
|
|
|
|
#5 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
Check this stuff
slide 4 in https://www.osti.gov/servlets/purl/1373464 Table at the end of this page for different fuels https://www.twi-global.com/technical...with%20propane. I think you are getting about the right number, IF you are using pure oxygen as oxidizer. And, once again, the flame shape is very reasonable in your contour, so I think it is a good CFD. Perhaps you have to converge it a little bit more, it should get a bit more symmetric and maybe the peak temperature will go down a bit |
|
|
|

|
|
|
|
|
#6 |
|
New Member
Ozan KEKÜL
Join Date: Nov 2020
Posts: 8
Rep Power: 5  |
Hello Lorenzo,
Yes i use pure oxygen as the oxidizer and there is not any nitrogen in the oxidizer. As far as i understand, you say this result is acceptable. I will also model methane/oxy and NH3/air mixtures with same model combustor and compare the results. By the way i will read the files which you have sent. Thanks for your support. On the other hand, according to my searching in the literature, Cp (specific heat) values of the species is very important to predict good temperature profiles. And also A value in the reaction dialog box in eddy dissipation model. Maybe i will have to fix these cp and A values. there are some articles which say 1 is more proper selection for A values and i will have to optimize cp values between 300 3000 K for all species (there is a picture in here). After i have run a new model for methane/oxy combustion and nh3/air combustion , i will inform. Thanks. 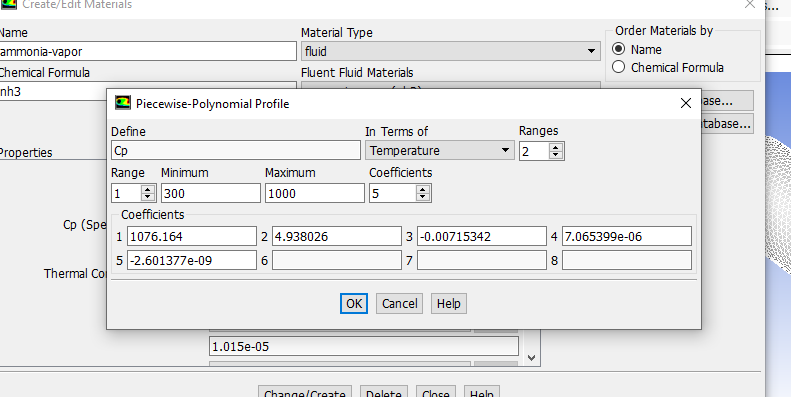
|
|
|
|

|
|
|
|
|
#7 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
I would leave the Cp values in polynomial form with the default values. Those are well enstablished ones, do not touch them. Here you are visualizing the Cp polynomial between 300 and 1000, but if you click on the arrows near Range on the left it will show you the coefficents for the other temperature range
Considering the A value, with respect to what do you expect to tune it? Do you have some experimental data on what you are simulating? If not, I don't see any point in tuning parameters |
|
|
|

|
|
|
|
|
#8 |
|
New Member
Ozan KEKÜL
Join Date: Nov 2020
Posts: 8
Rep Power: 5  |
Regarding to A value, there is an article named "The model constant A of the eddy dissipation model " by Peiyong Wang. In this article, the author has tried to find optimal A value. but there is not any data for NH3 / oxy flame. I thought that maybe i can improve the predicted temperature value according to this information. If you read this article , i wonder your comments.
By the way, my methane/oxy model is running right now. When i get the results , i will compare the results of nh3/oxy and ch4/oxy flame. if the axial temperature value of the NH3 / oxy flame is higher then CH4/oxy flame, there is a problem. Do you agree with that ? Regarding to B value, only thing that i know is this value is considered for premixed flames. Since my flame is diffusion , i dont know should i change this value. But i dont have any information. After i had got the result of ch4/oxy combustion and if the temperature is lower than NH3/oxy, i will try to find another solution by changing these values. On the other hand, i know that eddy dissipation model predicts higher temperature values than PDF model etc. but i have to find a way a way to optimize temperature predictions i EDM because, i have model nox formation and as far as i know there is not any possbility to predict fuel nox in PDF model. Indeed the reason i choosed eddy dissipation model for fuel nox prediction. Do you have any idea about that ? |
|
|
|

|
|
|
|
|
#9 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
What I am saying is that usually you tune the model parameters to match experimental data, if you don't have experimental data of your combustor, you have no reason to tune the model parameters, because, basically, what would you tune them against?
What people usually do in companies ( I was working for GE Aviation) is tune whatever model they want to use agains an experimental database they have, then use it to predict the flame characteristics in new geometries. If you don't have such experimental data bank, it makes no sense to tune parameters. When you talk about improving flame prediction, improving with respect to what? Yes, I expect that if you use air as oxidizer the temperature will be lower. I remember running NOx predictions with Partially premixed model, no problem with that. |
|
|
|

|
|
|
|
|
#10 |
|
New Member
Ozan KEKÜL
Join Date: Nov 2020
Posts: 8
Rep Power: 5  |
Yes, you are right. Indeed, we have the experiment rig in our laboratory but we dont have the gases yet. When we will have the gases ,our experimental studies will begin.
According to my searches, Mixture Fraction/PDF model has showed good match with experimental data but i could not find how to include fuel nox prediction in this model. Since NH3 has nitrogen, fuel nox must be taken into account. I have not any experience with partially premixed model, but do you know how to predict, all nox distributions (fuel, prompt, zeldovich) in Mixture Fraction/PDF model? Thanks for your support by the way. |
|
|
|

|
|
|
|
|
#11 |
|
Senior Member
Lorenzo Galieti
Join Date: Mar 2018
Posts: 373
Rep Power: 12  |
Never had any experience with nitrogen containing fuels... For me it was alwais kerosene.
I am sure the partially premixed model (not the premixed one) can be used with NOx, because that is what I ran a couple of times. (I cannot grant on accuracy though). Concerning the guidelines, unfortunately I have nothing. |
|
|
|

|
|
 |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Problem in modified pisoFoam with temperature equation + thermophysical model | Matt_B | OpenFOAM Programming & Development | 17 | November 7, 2019 09:23 |
| problem with convergence in combustion model ? | sooraj546 | FLUENT | 0 | September 14, 2019 10:20 |
| Eddy dissipation model combustion flame temp much higher than theoritical | shaman | CFX | 5 | October 20, 2016 20:52 |
| Wrong flow in ratating domain problem | Sanyo | CFX | 17 | August 15, 2015 06:20 |
| temperature too high in eddy dissipation | Peter Lemtis | FLUENT | 3 | November 24, 2002 00:45 |