 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
New Member
Join Date: Nov 2023
Posts: 2
Rep Power: 0  |
Is there a proof for why the stagnation conditions (
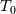 and and 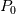 ) may be used in the Tds equations? ) may be used in the Tds equations?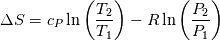 Denton 1993 states "the temperatures, pressures, and densities used in the Tds equations may be either all static values or all stagnation values because by definition the change from static to stagnation conditions is isentropic." Further, some sources justify this with "the Tds equations apply between any two thermodynamic end states". Are stagnation temperature and stagnation pressure thermodynamic state variables? |
|
|
|

|
|
|
|
|
#2 |
|
Senior Member
Filippo Maria Denaro
Join Date: Jul 2010
Posts: 6,771
Rep Power: 71    |
Quote:
This is a jump relation for the entropy in the normal shock wave, being adiabatic the stagnation temperature is constant but not the stagnation pressure. The states are determined indipendently from the path. |
|
|
|

|
|
|
|
|
#3 |
|
New Member
Join Date: Nov 2023
Posts: 2
Rep Power: 0  |
||
|
|

|
|
|
|
|
#4 | |
|
Senior Member
Filippo Maria Denaro
Join Date: Jul 2010
Posts: 6,771
Rep Power: 71    |
Quote:
Yes, in general you start from Gibbs relation to integrate between the state 1 and 2. |
||
|
|

|
||
|
|
|
#5 |
|
Senior Member
Lucky
Join Date: Apr 2011
Location: Orlando, FL USA
Posts: 5,675
Rep Power: 66    |
The key assumption is that the the process going from the static to stagnation conditions is via an isentropic process.
The entropy change from 1 to 01 is 0 because the change from static-to-stagnation conditions is (assumed to be) isentropic. The change from 2 to 02 is also 0. Hence the change from 1 to 2 is equal to the change from 10 to 20. Recall also that for a simple system, the thermodynamic state is fully characterized by two intensive properties (static temperature and static pressure). The stagnation conditions at state 1 or 2 are fully specified by knowing their static conditions and vice versa, hence the entropy change between 1 and 2 or 01 and 02 must be equal or you do not have a thermodynamic system. It is common in engineering applications to assume that the change from static to a (hypothetical) stagnation conditions is isentropic. This condition is explicitly noted in your quote of Denton. The usage of stagnation properties for performance calculations in turbomachinery is also consistent with this assumption. |
|
|
|

|
|
 |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Fortran - Lid-Driven Cavity Boundary Conditions Error when using SIMPLE method | BaklavaBaby29 | Main CFD Forum | 8 | January 23, 2023 17:22 |
| Radiation in semi-transparent media with surface-to-surface model? | mpeppels | CFX | 11 | August 22, 2019 07:30 |
| Periodic boundary conditions for compressible Euler equations | selig5576 | Main CFD Forum | 24 | April 25, 2017 16:43 |
| Exit Boundary Conditions N-S equations | lavan | Main CFD Forum | 2 | July 2, 2005 03:10 |
| A problem about setting boundary conditions | lyang | Main CFD Forum | 0 | September 19, 1999 18:29 |